Phytoremediation of Copper in water using Indian Mustard Seed
Mustard isn’t just for topping hot dogs any more. Scientists at Brookhaven National Laboratory are using Indian mustard (Brassica juncea) and cabbage (Brassica oleracea) plants to clean up low level concentrations of cesium 137 and strontium 90 in the soil. A consortium sponsored by the Subsurface Contaminants Focus Area recently completed studies with a test plot using plants from the mustard family to take up these radioactive contaminants from the soil.
In New Jersey, Trenton’s Gould National Battery site was home to commercial lead acid battery manufacturers from the mid 1930s to the early 1980s. To assist the City in restoring this site, EPA awarded Trenton $200,000 to try a new soil cleanup technique in which plants are used to extract lead and other heavy metals from the ground. In the case of the Gould site, Indian mustard plants are being used.
In Hartford, Connecticut, a vacant city lot that had been polluted with toxic levels of lead (Pb) has been cleaned by Indian mustard planted at the site. In one summer, lead in the soil was reduced lead from levels in excess of 1,000 parts per million (p.p.m.) to the allowable level of fewer than 500 p.p.m. (Note: Parts per million (p.p.m.) is an expression of concentration which means one milligram of lead per kilogram of soil.)
Indian mustard plants exposed to lead while growing in water were able to accumulate up to 55 mmol/kg dry shoot tissue (the plant was dried out to remove the water it contained). Expressed in terms of weight, over 1% of the plant's mass was lead! This represents a 75 fold concentration of lead in plant tissue over that in solution.
In addition to lead, Indian mustard accumulates selenium, sulfur, chromium, cadmium, nickel, zinc, and copper during growth. Indian mustard has also been reported to accumulate heavy metals during germination.
Materials and Methods
To test the ability of Indian mustard to absorb copper, we designed a germination chamber that allowed the germinating seeds to be continuously bathed (but not submerged) in the water containing copper. We started with a small aquarium with a disposable plastic container, glued to the aquarium bottom, to serve as a support base for the germination chamber. (see Figure 1)
Figure 1

"We started with a small aquarium with a disposable plastic container, glued to the aquarium bottom, to serve as a support base for the germination chamber."
Figure 2

"The germination chamber was made from a disposable plastic dish with small drain holes placed in one end..."
Figure 3

"...two pieces of 3/4 inch PVC pipe glued into the other end."
The germination chamber was made from a disposable plastic dish with small drain holes placed (see Figure 2) in one end and two pieces of 3/4 inch PVC pipe glued into the other end (see Figure 3). The PVC pipes were long enough to reach within 5 cm of the aquarium bottom when the chamber was placed onto the support base. The tops of the PVC pipes reached one inch above the inside bottom the germination chamber.
The germination chamber was then placed on the support base and tilted toward the drain holes (see Figure 4).
Figure 4
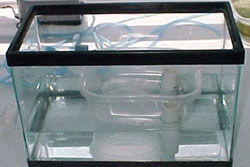
"The germination chamber was then placed on the support base and tilted toward the drain holes."
Figure 5

"Two air stones were then placed in the PVC pipes and attached to an air pump."
Figure 6

"The air stones in the PVC pipe release air bubbles which cause water to move to the top of the PVC pipes and out into the germination chamber."
Two air stones (see Figure 5) were then placed in the PVC pipes and attached to an air pump. The air stones in the PVC pipe release air bubbles (see Figure 6) which carry water to the top of the PVC pipes and out into the germination chamber (see Figure 7). (Note: air stones are readily available anywhere aquarium supplies are sold.)
Ten grams of Indian mustard seeds (see Figure 8) were allowed to germinate for 3-4 days in a small coffee filter with distilled water before being placed into the germination chamber. This was done to alleviate any stress from the heavy metal on the start of the germination process. Once the Indian mustard sprouted the coffee filter was placed into the germination chamber. Small holes were poked in the drain side of the coffee filter with a pin to insure that the water was not trapped in the coffee filter where it could drown the germinating seedlings.
A plastic cover was added to the aquarium to prevent water evaporation as well as any splattering of the heavy metal (see Figure 9).
Knowing the initial amount of water placed in the aquarium, enough copper sulfate solution (100 p.p.m.) was added to give a starting concentration of 20 p.p.m. The copper concentration in the aquarium water was measured daily for 5 days (see below for measurement techniques).
The amount of copper remaining after the test period was 4.6 p.p.m. Subtracting this from the initial concentration of 20 p.p.m. we find that 15.4 p.p.m. of copper has been absorbed by the mustard seeds. Multiplying 15.4 times the 3 liters of water in the aquarium gives us 46.2 milligrams of copper that has been removed. Dividing by the 10 grams of dry seeds we started with gives us 4.6 milligrams of copper removed per gram of germinating seeds. This is equivalent to 460 grams of copper that would be removed by 220 pounds of germinating mustard seeds.
Figure 7

"The PVC pipes were long enough to reach within 2 inch of the aquarium bottom when the chamber was placed onto the support base."
Figure 8

"Indian mustard seed sprouts."
Figure 9

"A plastic cover was added to the aquarium to prevent water evaporation as well as any splattering of the heavy metal."
Measuring Copper Concentration

To measure the copper concentration in the water, one could use a visible light spectrophotometer or a test kit. The Chemets copper test kit by Chemetrics has been found to be fast and reliable. The sample is mixed with the reagent, and the resultant color is compared against provided colorimetric standards to determine the concentration of copper in a sample. See CHEMetrics Visual Comparison Kits and CHEMetrics Copper Test Kits for more details. A swimming pool supply company might also be able to recommend a source for a copper test kit.
This activity provided courtesty of the University of Florida's Center for Precollegiate Education and Training (CPET).